Lithium-Ion Batteries
 Lithium-Ion Battery Pack
Lithium-Ion Battery Pack
We hear a lot bout Lithium-Ion technology in the EV world.
The fact is most of the electric car manufacturers have moved away from the older Lead-Acid & Nickel-Metal Hydride (Ni-Mh) based chemistries and use Lithium based batteries. Although the cheaper e-bikes in India still use lead acid batteries to save costs.
You can find Lithium-ion batteries in laptops, PDAs, cell phones and iPods. They're very common because, they're some of the most energetic rechargeable batteries available, in other words, lithium-ion batteries can store more energy per kilogram as compared to Lead-Acid or Ni-Mh based batteries.
The fact is most of the electric car manufacturers have moved away from the older Lead-Acid & Nickel-Metal Hydride (Ni-Mh) based chemistries and use Lithium based batteries. Although the cheaper e-bikes in India still use lead acid batteries to save costs.
You can find Lithium-ion batteries in laptops, PDAs, cell phones and iPods. They're very common because, they're some of the most energetic rechargeable batteries available, in other words, lithium-ion batteries can store more energy per kilogram as compared to Lead-Acid or Ni-Mh based batteries.
Pros and Cons of Lithium-Ion chemistry
- They're generally much lighter than other types of rechargeable batteries of the same size. A lot of energy can be stored in Lithium's atomic bonds. This translates into a very high energy density for lithium-ion batteries. A typical lithium-ion battery can store 150 watt-hours of electricity in 1 kilogram of battery. A lead-acid battery can store only 25 watt-hours per kilogram. It takes 6 kilograms to store the same amount of energy that a 1 kilogram lithium-ion battery can handle. That's a huge difference.
- A lithium-ion battery pack loses only about 5 percent of its charge per month, compared to a 20 percent loss per month for NiMH batteries.
- Lithium-ion batteries can handle hundreds of charge/discharge cycles.
- They start degrading as soon as they are produced. They will only last up to five years from the date of manufacture whether you use them or not.
- They are extremely sensitive to high temperatures. Heat causes lithium-ion battery packs to degrade much faster than they normally would.
- A lithium-ion battery pack must have an on-board computer to manage the battery. This makes them even more expensive than they already are.
- Lithium-ion battery cells are sold in "battery packs", which include battery management systems (BMS).
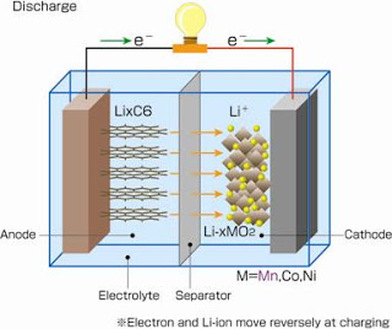 Movement of Lithium Ions
Movement of Lithium Ions
How does it work?
A lithium-ion battery cell contains four main components
The Cathode is usually made out of lithium metal oxide powder and they emit lithium-ion to anode during charging. They receive lithium-ion during discharge.
The Anode is made out of graphite powder, they receive lithium-ion during charging and they emit lithium-ion during discharge.
The electrolyte aids in transporting the lithium-ion between the Cathode and Anode. The separator prevents short circuit between Cathode and Anode and are usually made of micro-porous membranes.
The illustration on the right shows what happens during charging/discharging.
During discharge, in the Anode, the lithium atoms interface with the electrolyte and they lose an electron, which goes through he external circuit and at the same time the remaining lithium ion, enters into the electrolyte. Now on the same time at the Cathode, we have lithium ions entering the lithium metal oxide based Cathode. The electrons too arrive from the external circuit.
So basically this is the discharge cycle. Lithium ions moves from the Anode to the Cathode, along with the electrons that go through the external circuit. And when you recharge the battery, the reverse process happens - Lithium ions would leave the Cathode and move towards the Anode and a series of electrons move towards the Anode.
To bring bout this recharging process, we need to apply a voltage which is similar to the voltage that is applied during discharge.
Some people call this a 'rocking chair' battery - as the lithium ions rock between the Anode and Cathode.
A lithium-ion battery cell contains four main components
- Cathode
- Anode
- Electrolyte
- Separator
The Cathode is usually made out of lithium metal oxide powder and they emit lithium-ion to anode during charging. They receive lithium-ion during discharge.
The Anode is made out of graphite powder, they receive lithium-ion during charging and they emit lithium-ion during discharge.
The electrolyte aids in transporting the lithium-ion between the Cathode and Anode. The separator prevents short circuit between Cathode and Anode and are usually made of micro-porous membranes.
The illustration on the right shows what happens during charging/discharging.
During discharge, in the Anode, the lithium atoms interface with the electrolyte and they lose an electron, which goes through he external circuit and at the same time the remaining lithium ion, enters into the electrolyte. Now on the same time at the Cathode, we have lithium ions entering the lithium metal oxide based Cathode. The electrons too arrive from the external circuit.
So basically this is the discharge cycle. Lithium ions moves from the Anode to the Cathode, along with the electrons that go through the external circuit. And when you recharge the battery, the reverse process happens - Lithium ions would leave the Cathode and move towards the Anode and a series of electrons move towards the Anode.
To bring bout this recharging process, we need to apply a voltage which is similar to the voltage that is applied during discharge.
Some people call this a 'rocking chair' battery - as the lithium ions rock between the Anode and Cathode.
Power in a Lithium-Ion battery
Power is important, because when we drive an EV, typically we will have to accelerate for short periods of time, so getting that energy out quickly from a battery is quiet important. Usually there is a trade off between the power that can be extracted from a battery and the net energy that can be stored in the same battery.
Power is important, because when we drive an EV, typically we will have to accelerate for short periods of time, so getting that energy out quickly from a battery is quiet important. Usually there is a trade off between the power that can be extracted from a battery and the net energy that can be stored in the same battery.
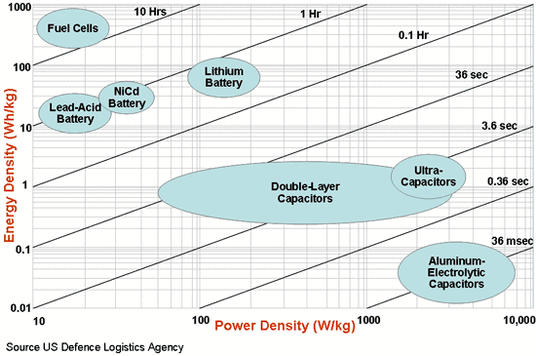 Ragone chart
Ragone chart
On the right, you can see a Ragone chart which is used for performance comparison of various energy storing devices. On such a chart the values of energy density (in Wh/kg) are plotted versus power density (in W/kg).
Conceptually, the vertical axis describes how much energy is available, while the horizontal axis shows how quickly that energy can be delivered, otherwise known as power, per unit mass.
The sloping lines on the Ragone plots indicate the relative time to get the charge in or out of the device.
From the chart, we can deduce that that ultracapacitors (supercapacitors) can deliver very high power but the storage capacity is very limited. On the other hand Fuel Cells can store large amounts of energy but have a relatively low power output. Lithium batteries are somewhere in between and provide a reasonable compromise between the two.
Even though Lithium-Ion batteries offer a good compromise between energy and power, we need to have a battery which can have better energy storage capacity with higher power ratings.
Conceptually, the vertical axis describes how much energy is available, while the horizontal axis shows how quickly that energy can be delivered, otherwise known as power, per unit mass.
The sloping lines on the Ragone plots indicate the relative time to get the charge in or out of the device.
From the chart, we can deduce that that ultracapacitors (supercapacitors) can deliver very high power but the storage capacity is very limited. On the other hand Fuel Cells can store large amounts of energy but have a relatively low power output. Lithium batteries are somewhere in between and provide a reasonable compromise between the two.
Even though Lithium-Ion batteries offer a good compromise between energy and power, we need to have a battery which can have better energy storage capacity with higher power ratings.
Technology & cost challenges
Current battery performance of lithium-ion batteries is sufficient to be used in EVs or PHEVs, but still there is a need to increase in energy and power density. Ather improvements needed are namely - Durability, Safety and Cost.
Current battery performance of lithium-ion batteries is sufficient to be used in EVs or PHEVs, but still there is a need to increase in energy and power density. Ather improvements needed are namely - Durability, Safety and Cost.
- Durability
Cars tend to last around a decade or longer unlike consumer electronics. The problem of battery degradation in EVs is a major problem. We dont want to replace an expensive battery and if we have a significant reduction in range, we will loose out on the benefits of EV's.
Over time the battery degrades in a number of ways that can affect both power and capacity until eventually it simply can’t perform its basic functions. The lifespan of a lithium-ion pack depends mainly on the battery’s temperature, state of charge and charge habits. Battery performance begins to suffer as soon as the temperature climbs above 35 degrees.
A temperature above 35 degrees C affects the battery pack performance instantly and even permanently if it lasts many months.
In addition the power of lithium-ion batteries decreases in cold weather. The wrong charging techniques can also shorten a battery’s life. Lithium-ion battery packs need to stay as close as possible to a 50 percent charge, usually going no higher than 80 percent and no lower than 20 percent. Also its a good practice to refrain from doing too many “fast charges,” in which an EV battery can be recharged in under an hour.
Battery degradation mechanisms are complicated, they occur due to multiple chemical processes that occur within the battery. One case of degradation, forms in the Anode side of the battery. As recollected, the Anode is made out of a graphite material. At the edge of the Anode, a Solid Electrolyte Interface (SEI) is formed, typically during the first few charging cycles, due to a reaction between electrolyte and the Anode. Once the SEI is formed, we are loosing capacity as there is a conversion of the liquid electrolyte into a Solid SEI, which inhibits ion transport from the Anode to the Cathode.
The effects can get worse with increase in temperature, which increase the rate of SEI formation. The SEI also inhibits the power rate as ions cant move within the electrolyte freely.
If we operate the battery at low temperatures and at a high rate, lithium which is migrating through the SEI, can form solid dendrites. These are solid things that can pierce through the separator and can result in a short circuit in the battery.
These are some of the degradation mechanisms associated with Lithium-Ion batteries. Lithium-ion battery packs in EVs comes with their own BMS, cooling/heating systems etc, which could help prolong the durability of the batteries.
But still, further development will be necessary to overcome this problem. - Safety
Lithium-ion batteries are vulnerable to short-circuiting and overcharging. Lead Acid, Ni-Mh batteries perform safely even after short circuiting and overcharging because they have low energy capacity. However, when a lithium-ion battery short circuits, high electricity flows are created and the battery temperature increase to several hundred degrees, heating up neighboring cells, which results in battery combustion reaction. When this occurs the chemical structure of the Anode and Cathode are destroyed which makes the battery useless.
It is important to note that these situations are very rare. Still, it only takes a couple of fires and a little media coverage to prompt a recall. To prevent overcharging, lithium-ion batteries usually are sold as battery packs with very precise voltage control systems and temperature control systems. - Cost
According to most recent studies, the cost of lithium-ion batteries for vehicle use, is 4 to 8 times that of lead acid and 1 to 4 times that of Ni-Mh. Howwever the cost of lithium batteries is expected to decrease significantly as the batteries will be increasingly used for many applications, such as UPS, consumer electronics and backup power supplies. As the market grows, the production scales up and manufacturers will be able to enjoy economies of scale. According to studies the cost of lithium-ion batteries will decrease from $550/kWh in 2012 to $325/kWh in 2020.
The future
The batteries of the future must be smaller, lighter, less expensive and last longer.
Research is going on to improve lithium-ion batteries for example, we can make the lithium batteries which can have higher energy density by replacing the electrode materials by things which have higher capacity for Lithium. We can also swap out the electrolyte which can support operation at higher voltages - as voltage * capacity = higher energy. There are also solid state batteries, its like a lithium-ion battery, where the liquid electrolyte is replaced by a solid phase and the challenge there would be to find a solid material which can transport lithium-ions fast enough. The advantage of the solid state batteries is that we can have a robust and safer battery.
The batteries of the future must be smaller, lighter, less expensive and last longer.
Research is going on to improve lithium-ion batteries for example, we can make the lithium batteries which can have higher energy density by replacing the electrode materials by things which have higher capacity for Lithium. We can also swap out the electrolyte which can support operation at higher voltages - as voltage * capacity = higher energy. There are also solid state batteries, its like a lithium-ion battery, where the liquid electrolyte is replaced by a solid phase and the challenge there would be to find a solid material which can transport lithium-ions fast enough. The advantage of the solid state batteries is that we can have a robust and safer battery.
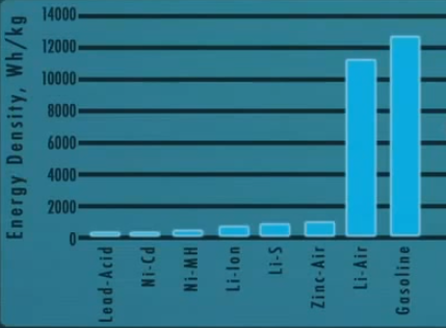 Energy density of Lithium-Air battery approaching the energy of Petrol
Energy density of Lithium-Air battery approaching the energy of Petrol
Research is also being conducted on the Lithium-Air or Lithium-Suphur batteries, which have among the highest potential specific energy density as compared to other technologies.
The lithium-air battery, Li-air for short, is a metal-air battery chemistry that uses the oxidation of lithium at the anode and reduction of oxygen at the cathode to induce a current flow. The major appeal of the Li-air battery is the extremely high energy density. Look at the graph on the right.
The blue bars are the energy density of various different battery chemistries and also gasoline. The Lithium-Air chemistry starts to approach the energy density of oil, which is phenomenal.
Lithium-air batteries can theoretically deliver 12 kWh per kg.
Just think bout it, the Mahindra e2o electric car has a 10kWh lithium-ion battery pack which weighs more than 200kg. A lithium-air battery pack in an electric car can increase the range of an EV significantly.
However, the lithium-air battery has never met with as much success as lithium-ion cells due to the difficulty of building a lithium-air cell which can be recharged thousands of times. Researchers have their doubts and acknowledge that it could be a decade before lithium-air batteries are a commercial reality.
Conclusion
Lithium-Ion batteries are the current state of the art technology for electric vehicles. However with so much research being made, we should not be surprised if a new chemistry or a modification of the Lithium-Ion cell will give us better energy density in our batteries which will lead to higher range, better durability and safety. When that happens, EVs will have a much better chance to compete against oil based vehicles in terms of power and energy density.
The lithium-air battery, Li-air for short, is a metal-air battery chemistry that uses the oxidation of lithium at the anode and reduction of oxygen at the cathode to induce a current flow. The major appeal of the Li-air battery is the extremely high energy density. Look at the graph on the right.
The blue bars are the energy density of various different battery chemistries and also gasoline. The Lithium-Air chemistry starts to approach the energy density of oil, which is phenomenal.
Lithium-air batteries can theoretically deliver 12 kWh per kg.
Just think bout it, the Mahindra e2o electric car has a 10kWh lithium-ion battery pack which weighs more than 200kg. A lithium-air battery pack in an electric car can increase the range of an EV significantly.
However, the lithium-air battery has never met with as much success as lithium-ion cells due to the difficulty of building a lithium-air cell which can be recharged thousands of times. Researchers have their doubts and acknowledge that it could be a decade before lithium-air batteries are a commercial reality.
Conclusion
Lithium-Ion batteries are the current state of the art technology for electric vehicles. However with so much research being made, we should not be surprised if a new chemistry or a modification of the Lithium-Ion cell will give us better energy density in our batteries which will lead to higher range, better durability and safety. When that happens, EVs will have a much better chance to compete against oil based vehicles in terms of power and energy density.
